
Color code: red = O, blue = N, gray = C, white = H.
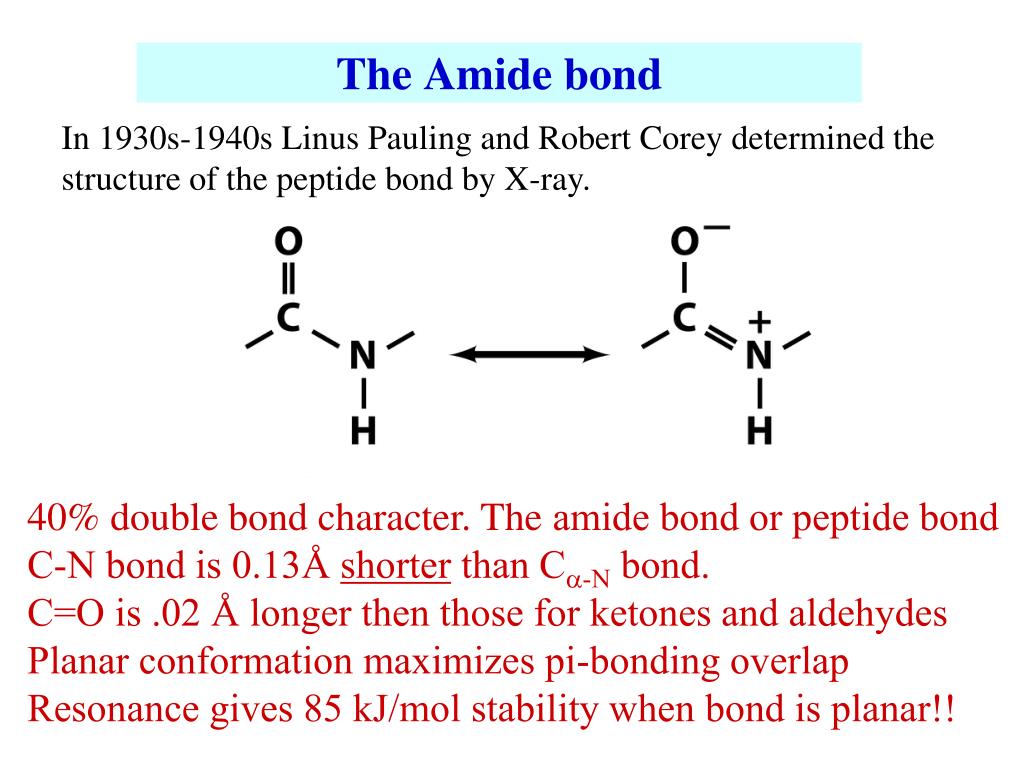
Structure and bonding Structure of acetamide hydrogen-bonded dimer from X-ray crystallography. Low-molecular-weight amides, such as dimethylformamide, are common solvents. Amides include many other important biological compounds, as well as many drugs like paracetamol, penicillin and LSD. Proteins and important plastics like Nylons, Aramid, Twaron, and Kevlar are polymers whose units are connected by amide groups ( polyamides) these linkages are easily formed, confer structural rigidity, and resist hydrolysis. Nomenclature Īmides are pervasive in nature and technology. Some uncommon examples of amides are N-chloroacetamide ( H 3C−C(=O)−NH−Cl) and chloroformamide ( Cl−C(=O)−NH 2).Īmides are qualified as primary, secondary, and tertiary according to whether the amine subgroup has the form −NH 2, −NHR, or −NRR', where R and R' are groups other than hydrogen. It can be viewed as a derivative of a carboxylic acid ( R−C(=O)−OH) with the hydroxyl group ( −OH) replaced by an amine group ( −NR′R″) or, equivalently, an acyl (alkanoyl) group ( R−C(=O)−) joined to an amine group.Ĭommon of amides are formamide ( H−C(=O)−NH 2), acetamide ( H 3C−C(=O)−NH 2), benzamide ( C 6H 5−C(=O)−NH 2), and dimethylformamide ( H−C(=O)−N(−CH 3) 2). The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula R−C(=O)−NR′R″, where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. General structure of an amide (specifically, a carboxamide) Formamide, the simplest amide Asparagine ( zwitterionic form), an amino acid with a side chain (highlighted) containing an amide group


 0 kommentar(er)
0 kommentar(er)
